Clinical Oncology Research Journal
ISSN 2652-4457
Case Report
Clinical Efficacy of Ponatinib in Philadelphia Positive T-Cell Acute Lymphoblastic Leukemia with Extramedullary Involvement
Gianluca Cristiano*, Jacopo Nanni, Chiara Sartor, Sarah Parisi, Giovanni Marconi, Francesco Barbato, Mario Arpinati, Francesca Bonifazi, Antonio Curti, Michele Cavo, Stefania Paolini# and Cristina Papayannidis#
Institute of Hematology/Oncology “L. And A. Seràgnoli” S.Orsola-Malpighi Hospital, University of Bologna, Italy
*Corresponding author: Gianluca Cristiano, Institute of Hematology/Oncology “L. And A. Seràgnoli” S. Orsola-Malpighi Hospital, University of Bologna, Italy, Tel: +39 3803033717; E-mail: gianluca.cristiano2@studio.unibo.it
Citation: Cristiano G, Nanni J, Sartor C, Parisi S, Marconi G, et al. (2019) Clinical Efficacy of Ponatinib in Philadelphia Positive T-Cell Acute Lymphoblastic Leukemia with Extramedullary Involvement. Clin Oncol Res J: CORJ-100009.
Received date: 30 October, 2020; Accepted date: 17 November, 2020; Published date: 24 November, 2020
#equally contributed
Abstract
T- cell Acute Lymphoblastic Leukemia (T-ALL) is a rare entity in adult acute leukemia setting. Translocation (9;22) (q34;q11) and BCR-ABL1 rearrangement are occasionally found in T-ALL and have been reported in no more than 100 cases in literature (most of which are CML blast crisis). Here, we report the remarkable effectiveness of third-generation Tyrosine-Kinase Inhibitor (TKI) ponatinib in obtaining hematological and metabolic remission, in a patient with Philadelphia (Ph) chromosome positive de novo T-ALL, and outcomes of a therapeutic strategy containing chemotherapy intensification, nelarabine and allogeneic hematopoietic stem cell transplantation.
Keywords: Philadelphia chromosome; Ponatinib; T-cell Acute Lymphoblastic Leukemia
Introduction
A 60 years-old man was diagnosed with Ph+ T-ALL, after a 2 months history of right-sided persistent painless neck lymphadenopathies, without fever, weight loss or other systemic symptoms. The lymph node biopsy was diagnostic for T-ALL being CD3+, PAX5-, TdT+, CD4-/+, CD8+; Ki-67 index was 80% at immunofluorescence.
Peripheral blood count at diagnosis showed total leukocyte count 15.2 x 10^9/L (20% lymphoblasts), hemoglobin 120 g/L, platelets 231 x 10^9/L. The bone marrow (BM) smear revealed increased cellularity with 40% lymphoid blast cells and flow cytometry was positive for CD2, CD5, CD7, CyCD3, with CD33 aberrant expression and negative for CD34 and CD1a. Cytogenetic analysis reported 46XY, t(9;22) (q34;q11)[9]/47XY,t(9;22)(q34;q11),+19[4]/46XY[14] and FISH confirmed BCR-ABL1 rearrangement (62.5%). BCR-ABL1 RT-PCR revealed p190 fusion transcript, with BCR-ABL1/ABL1x100 = 11.72. We then researched BCR-ABL1 transcript also on the diagnostic lymph node that, interestingly, revealed to be positive (BCR-ABL1/ABL1x100 = 132.39), thus confirming the concordance between BM and extramedullary disease. No patient-specific V(d)J rearrangement was identified, which did not allow us to monitor molecular disease besides the use of BCR-ABL1 RT-PCR either. No Central Nervous System (CNS) disease was detected at lumbar puncture (LP). A total body 18-FDG PET/CT showed multiple lymphadenopathies located on both sides of the diaphragm (Figures A1, B1 and C1, SUV max 5.6 in the neck).
In such a rare entity, a standard of care is not established. Therefore, following institutional policy and preliminary results of first line therapy with ponatinib within a prospective phase II study, demonstrating the efficacy of a TKI-based chemo-free approach [1], ponatinib 45 mg/die in combination with prednisone 1 mg/kg was started. Cardiovascular screening was performed before treatment and no contraindications to ponatinib were documented. Prednisone was tapered and stopped at day 28. Monthly, prophylactic medicated LPs were administered. The treatment was well tolerated without any relevant Adverse Events (AEs). After about 12 weeks of therapy, a 18-FDG PET/CT was performed, showing a complete normalization of all lymph node localizations (Figure A2 and B2). The BM evaluation resulted in complete morphological (Figure C2) and cytogenetic remission, with persistence of minimal Molecular Residual Disease (MRD) with BCR-ABL1/ABL1x100 = 0.35.
In order to intensify treatment, standard chemotherapy according to Hyper-CVAD regimen was added [2]. The patient received two Hyper-CVAD cycles: G3 febrile neutropenia occurred during both cycles and time to hematological recovery was analogous to clinical data [2]. After the first course (day 110 from diagnosis), the molecular evaluation showed a persistent MRD positivity (BCR-ABL1/ABL1x100 = 0.11), which was maintained after the second course (BCR-ABL1/ABL1x100 = 0.14). The disease revaluation with 18-FDG PET/CT confirmed the complete metabolic remission status. In the meantime, based on the high-risk features of the disease and the persistence of MRD, the search for a stem cell donor was started and a bridge-to-transplant strategy was built up. In this scenario, in ALL patients the presence of molecular disease is a major indication for allogeneic Hematopoietic Stem Cell Transplant (HSCT), but it is widely considered a poor prognostic factor for post-transplant outcome [3]. Considering this issue, the patient was treated with a course of Nelarabine (at the dose of 1500 mg/mq/die on days 1,3 and 5), that is reported to be active in MRD+ T-ALL [4]. Ponatinib was discontinued during Nelarabine course and restarted four days later. Of note, after the Nelarabine course BCR-ABL1 transcript had remarkably reduced and this lower level was maintained upon subsequent ponatinib treatment awaiting HSCT.
At day 250 from diagnosis, with 0.004 BCR-ABL1/ABL1x100 copies and no imaging signs of extramedullary involvement, the patient underwent allogeneic peripheral blood HSCT from HLA 10/10 matched unrelated donor. He received a Thiotepa-Busulfan-Fludarabine full conditioning regimen. GvHD prophylaxis comprised Anti-T-Lymphocyte Globulin (ATLG), methotrexate and cyclosporine. Two weeks after the PBSC infusion, we notified the occurrence of locomotor ataxia due to severe endangerment of lower limbs sensitivity associated with painful paraesthesias. Lumbar magnetic resonance (MRI) showed a minimal stenosis of L1-S1 spinal canal; after LP, analysis of cerebrospinal fluid (CSF) resulted in albumine-cytological dissociation. In the suspect of Guillain-Barré Syndrome, therapy with high doses intravenous immunoglobulins was started, without any success. Needle electromyography (EMG) didn’t reveal lower limbs alterations. Nerve conduction studies demonstrated a severe peripheral sensorial pathway alteration. Methylprednisolone 1 mg/Kg was started, then escalated, with a slow initial benefit on neurological symptoms, associated with physiatric rehabilitation.
The last disease evaluation (6 months after HSCT) resulted negative for BCR-ABL1 quantitative transcript.
Discussion
There are only few case reports in literature describing Philadelphia chromosome positive T-ALL. Only less than about 100 cases including both de novo Ph+ T-ALL and Ph+ T-ALL developed as blastic crisis of chronic myeloid leukemia (CML) have been reported until now. Hence the incidence, clinical behaviour, therapeutic and prognostic significance of this entity are unknown [5]. This case describes the manageable safety profile and the relevant activity of ponatinib, both as single-agent and in combination with standard chemotherapy, in Philadelphia positive T-ALL.
Another challenge is represented by the distinction between de novo Ph+ T-ALL from a similar pathological entity evolving as a blastic crisis of CML. It is unclear how the presence of BCR-ABL protein regulates the differentiation or the immunophenotypical lineage switch of leukemic cells. A previous history of CML, adult age, increased number of circulating granulocytic precursors, eosinophils and basophils, absence of bone marrow ALL involvement, extramedullary disease and the presence of non e1a2 BCR-ABL breakpoint favors as T-ALL originating as blastic crisis [6]. Our adult patient had no previous history of CML; he had active bone marrow involvement and presented a p190 BCR-ABL transcript; hence we may conclude for a de novo Ph+ T-ALL diagnosis.
Since Philadelphia positivity in ALL is associated with poor prognosis, allogeneic HSCT in first remission is recommended in young and fit patients [7]. The combination of chemotherapy regimen together with the efficacy of TKIs such as ponatinib is effective in achieving long-term remission in patients with newly diagnosed Ph-positive B-ALL. The MD Anderson Cancer Center phase 2 clinical trial enrolled 76 patients who received 8 cycles of chemotherapy according to Hyper-CVAD regimen and high-dose methotrexate and cytarabine; ponatinib was given orally at 45 mg/die for the first 14 days of cycle 1, then continuously at 45 mg for the subsequent cycles. The protocol was then amended to reduce ponatinib dose according to the achievement of a complete molecular response (83% of patients enrolled). The most common grade 3 or 4 adverse events were infections, increased transaminase, pancreatitis, hypertension, venous thromboembolic events and skin rash. The authors reported a 3-year CR rate of 83% with an OS of 76% [2]. In the rare setting of Philadelphia positive T-ALL, treatment strategies reported so far include hyper-CVAD alone, or in combination with TKIs (dasatinib), followed, for patients who obtained complete responses, by allogeneic HSCT [6]. Our decision was to begin therapy with single agent ponatinib, in order to initially avoid chemotherapy toxicity on a high disease burden. With ponatinib as single agent, our patient achieved complete morphological, cytogenetic and metabolic remission. The addition of chemotherapy regimen to ponatinib, as allowed by trials in Ph + B-ALL [2], was chosen to induce a deeper molecular response, in order to pursue the minimal disease status before allogeneic HSCT. Hence, despite the poor prognosis of T-cell Ph+ ALL in case reports and case series, we documented a complete response of the disease, similarly to what observed in B-cell Ph+ ALL.
In ALL presenting with BCR-ABL1 fusion gene, the MRD measured by PCR for BCR-ABL1 and the IgH/TCR rearrangement in PCR was concordant in most cases. Nevertheless, in a minority of the cases, the fusion gene was present also in the non-lymphoblast cells, suggesting a complex pathogenesis and that the BCR-ABL fusion might have occurred in an early progenitor cell [8]. Moreover, literature reports rare cases of childhood with ALL and subsequent late developing of Ph chromosome. In those children, the T-cell phenotype seemed to be more frequent [9]. Unfortunately, from the diagnosis bone marrow sample, no clone-type emerged for TCR rearrangement, neither with Next Generation Sequencing (NGS) analysis, in order to allow this kind of investigations.
The post-transplantation clinical course was characterized by a poorly understood neurological complication. The hypothesis we built up refers to the complex immunological environment that follows HSCT, where the known neurological toxicity of nelarabine, administered as a bridge to transplantation [10], might have played a causative role. A GVHD-like manifestation seemed less probable considering the absence of other signs and symptoms of cGVHD.
Conclusions
The case described is an extremely rare entity, whose clinical and prognostic features remain unknown. Only case reports can be found in literature. We reported the manageable safety profile and the relevant activity of ponatinib, both as single-agent and in combination with standard chemotherapy, in Philadelphia positive T-ALL. Furthermore, Nelarabine, sequentially combined with ponatinib, demonstrated to be promising as a bridge-to-transplant strategy in the poorly explored setting of pre-transplant MRD positive T-ALL, although the incidence of post-transplant neurological complications warrants further investigation. The diagnostic debate (de novo and blast phase) and the identification of the leukemic stem cell (PCR for BCR-ABL1 and TCR) are still ongoing issues, which might have clinical relevant implications and might help in choosing the best treatment protocol for the specific patient [11]. We may conclude that the presence of Ph chromosome still represents an essential therapeutic target, even when associated with rare disease characteristic such as the T-cell lineage. Moreover, investigating these entities, might help to gain insights into the pathogenesis of Ph positive acute lymphoblastic leukemias.
Conflict of Interest: Nothing to report.
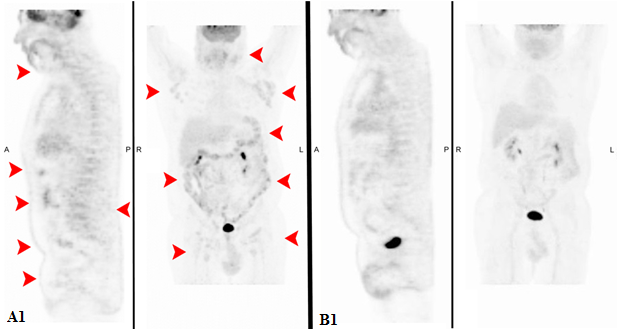
Figure A1-2: Comparison of total body 18-FDG PET/CT at onset and after ponatinib.
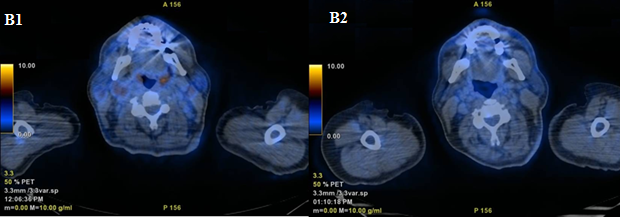
Figure B1-2: Another comparison of total body 18-FDG PET/CT at onset and after ponatinib.
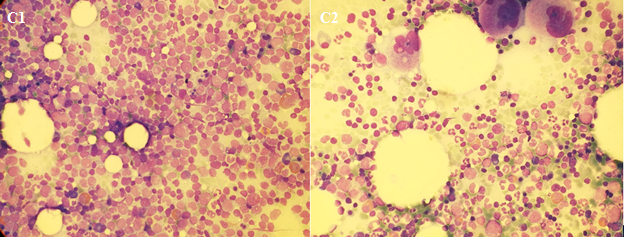
Figure C1-2: Bone marrow morphology at onset and after ponatinib.
Citation: Cristiano G, Nanni J, Sartor C, Parisi S, Marconi G, et al. (2019) Clinical Efficacy of Ponatinib in Philadelphia Positive T-Cell Acute Lymphoblastic Leukemia with Extramedullary Involvement. Clin Oncol Res J: CORJ-100009.